Which elements help with the growth, which help with flowering and fruiting, we have answers.
Plant nutrient elements are similar to the five food groups for human. Lacking any of them could cause problem and we can prevent them by providing those elements to the plants. There are sixteen essential nutrient elements for plants which can be divided into two groups as follows:
1.1 Essential elements which can be gained from water and atmosphere: carbon (C), oxygen (O), and hydrogen (H)
1.2 Essential elements which can be insufficient in the soil and need to be provided through fertilization: nitrogen (N), phosphorus (P) and potassium (K)
1.3 Elements less important than the essential elements which are found quite enough in the soil but should be monitored for plantation of over three years where fertilization is recommended: calcium (Ca), magnesium (Mg) and sulfur (S)
The seven less essential elements which can be insufficient in the soil: iron (Fe), manganese (Mn), boron (B), molybdenum (Mo), copper (Cu), zinc (Zn) and chlorine (Cl)
The Main Benefits of Each Nutrient Element
| Elements | Roles and Benefits |
| Nitrogen (N) | – stimulates growth and gives the plants strength
– increases proteins – increases greenness and helps growing leaves and trucks |
| Phosphorus (P) | – stimulates growth for the roots
– stimulates maturity – stimulates flowering, fruiting and seeding – increases immunity toward diseases |
| Potassium (K) | – helps the plants synthesize starch and sugar
– helps moving starch and sugar from the leaves to the fruits and the roots in root crops – helps improve the quality and taste of the fruits – helps increase immunity toward diseases and insects – control the breathing system and the opening and closing of the stomas |
| Calcium (Ca) | – is the main component of the plant’s cell walls and is necessary for the plant cells division
– works with boron in pollination – helps growing roots and leaves – helps moving starch, sugar and proteins |
| Magnesium (Mg) | – is the main component of chlorophyll (greenness in plants)
– helps generating proteins, fat, vitamins and sugar – encouraging phosphorus delivery to the trunks – balancing cell pH |
| Sulfur (S) | – generates amino acids, proteins and vitamin B
– helps generating colors, scent and oil – helps with chlorophyll synthesis |
| Iron (Fe) | – helps with chlorophyll synthesis
– has an important role in photosynthesis and breathing cycles |
| Manganese (Mn) | – helps with photosynthesis
– stimulates enzyme functions |
| Boron (B) | – encourages flowering
– helps with pollination and fruiting – helps the plants to use nitrogen and calcium more efficiently – helps transporting plant hormones |
| Molybdenum (Mo) | – helps with protein synthesis
– helps the plants to use nitrogen more efficiently |
| Copper (Cu) | – helps with chlorophyll synthesis
– stimulate enzyme functions – plays a role in breathing and protein and starch utilization |
| Zinc (Zn) | – helps generating chlorophyll and starch
– helps generating oxygen hormones which helps with stalk elongation |
| Chlorine (Cl) | – helps generating certain hormones
– helps stimulating maturity |
Apart from the above-mentioned elements, there are some other elements which contribute to growth stimulation in plants, (which Assistant Professor Yongyuth Osotspa defined as “contributing elements”), including the following two elements:
– helps increasing immunity toward diseases and less chance of breaking
– increases rice produce
– substitutes potassium (K) in some cases
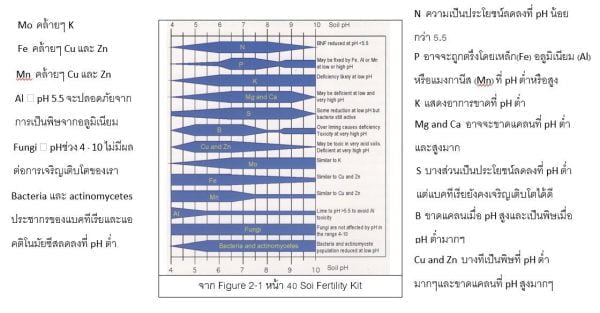
From figure 2-1, page 40: Soil Fertility Kit
– The benefits of nitrogen (N) decrease when pH is below 5.5.
– Phosphorus (P) might be fixated by iron (Fe), aluminum (Al) or manganese (Mn) when pH is too high or too low.
– Potassium (K) lacking will show when the pH is low.
– Magnesium (Mg) and calcium (Ca) might be insufficient when pH is very high or very low.
– Part of the benefits of sulfur (S) decreases at lower pH, while bacteria continue to grow.
– The lack of boron (B) occurs when the pH is high, while the element becomes toxic when the pH is very low.
– Sometimes copper (Cu) and zinc (Zn) become toxic when the pH is very low and insufficient at very high pH.
– Molybdenum (Mo) shares similar functions as potassium (K).
– Iron (Fe) and manganese (Mn) share similar functions as copper (Cu) and zinc (Zn).
– Aluminum (Al) toxicity is not present at pH 5.5.
– Fungi growth is not affected at pH 4-10.
– Bacterial and actinomycete population increase at lower pH.
Factors to Monitor from Soil pH
| Factors | Effects |
| Aluminum toxicity | Aluminum toxicity decreases as soil pH increases |
| Benefits of phosphorus | The benefits of phosphorus are high at soil pH 5.5-7.0 |
| Benefits of micronutrient elements | At pH 5.5-6.0, micronutrient element could highly be beneficial except molybdenum (Mo), (manganese and iron become less toxic at pH 5.5-6.0) |
| Ionization ability of elements with positive ions such as calcium (Ca), magnesium (Mg), potassium (K) | A condition of soil with higher pH increasing the ionization ability of elements with positive ions, meaning the soil has more ability to keep calcium (Ca), magnesium (Mg), potassium (K) before they are washed away |
| Processes where nitrogen (N) is released from organic compounds in a beneficial form for plants | The processes where nitrogen is released from organic compounds in a beneficial form occurs the most when the pH is around 5.5-6.5 |
| Nitrogen fixation from atmosphere | Nitrogen fixation from bean root nodules occurs less at pH below 5 |
| Plant diseases | Some of the plant diseases can be controlled by managing the soil pH, such as flaky skin in potatoes will decrease when the pH is higher |
| Phosphate rocks dissolvement | The soil pH must be less than 5.5 for phosphate rocks to dissolve and release phosphorus for plants to absorb |
From table 2-2, page 41: Soil Fertility Kit

From figure 3-2a, page 94: Soil Fertility Kit

From figure 3-2b, page 94: Soil Fertility Kit

From figure 3-2c, page 94: Soil Fertility Kit
When plants lack nutrients, following symptoms will be visible:
| Nutrient Elements | Occurrences on Plants | Pale Leaves | Dried Edges of Leaves | Colors and Shapes of the Leaves |
| Nitrogen (N) | Both younger and older leaves | Yes | No | Yellow on the leaves and veins |
| Phosphorus (P) | Older leaves | No | No | Purple lines on the leaves |
| Potassium (K) | Older leaves | Yes | Yes | Yellow areas on the leaves |
| Magnesium (Mg) | Older leaves | Yes | No | Yellow areas on the leaves |
| Calcium (Ca) | Younger leaves | Yes | No | Disfigured leaves |
| Sulfur (S) | Younger leaves | Yes | No | Yellow young leaves |
| Manganese (Mn) and iron (Fe) | Younger leaves | Yes | No | Pale colors between the veins |
| Boron (B), zinc (Zn), Copper (Cu), calcium (Ca) and molybdenum (Mo) | Younger leaves |
– |
– |
Disfigured young leaves |
From table 3-8, page 93: Soil Fertility Kit
Solutions to Fixing Malnutrient in Plants
Nitrogen – Fertilize with nitrogenous fertilizer by considering the following:
– In cases of acidic soil, use urea fertilizer (46-0-0) or ammonium nitrate (34-0-0) or calcium ammonium nitrate (27-0-0 + 5MgO + 7CaO).
– If the plants also lack sulfur, use ammonium sulfate (21-0-0 + 24S).
Phosphorus – Fertilize with phosphoric fertilizer by considering the following:
– If the plants only lack phosphorus, use triple super phosphate (0-45-0) or ammonium phosphate (11-52-0) or diammonium phosphate (18-46-0).
– In cases of acidic soil, use phosphate rocks (0-3-0 + 25CaO), which contain about 20-40% phosphorus (P2O5), completely dissolvent when soil pH is under 5.
Potassium – Fertilize with potassium fertilizer by considering the following:
– If the soil lacks potassium and the subject plants are tolerable to chloride, use potassium chloride (0-0-60 + 47Cl).
– When lacking potassium and nitrogen and the subject plants are intolerable to chloride, use potassium nitrate (13-0-46).
– When lacking potassium and sulfur and the subject plants are intolerable to chloride, use potassium sulfate (0-0-50 + 18S).
Calcium – Use a fertilizer or soil application containing calcium by considering the following:
– For highly acidic soil that also lacks phosphate, use phosphate rocks (0-3-0 + 25CaO), which contain 20-40% phosphate (P2O5), completely dissolvable when soil pH is under 5.
– In cases of non-acidic soil where phosphate is needed, use single super phosphate (0-22-0 + 28CaO + 11S) or triple super phosphate (0-46-0 + 12 CaO + 1.5 S)
– In cases of acidic soil only lacking calcium, use marl, which contains about 30% calcium (CaO).
– In cases of acidic soil and the plants also lack magnesium, use dolomite, which contains about 30% calcium (CaO) and 20% magnesium (MgO).
Magnesium – Use a fertilizer or soil application containing magnesium by considering the following:
– In cases of acidic soil where the plants usually lack calcium, use dolomite.
– In cases of non-acidic soil where sulfur is needed, use glyceride, which contains 27% magnesium (MgO) and 22% sulfur (S).
– In cases of non-acidic soil where potassium and sulfur are needed, use langbeinite, which contains 18% magnesium (MgO) and 22% sulfur (S) and 22% potassium (K2O).
Sulfer – Use a fertilizer or soil application containing sulfur nutrient by considering the following:
– If you wish to add nitrogen, use ammonium sulfate, which contains 21% nitrogen (N) and 24% sulfur (S).
– If you wish to add potassium, use potassium sulfate, which contains 50% potassium nutrient (K2O) and 18% sulfur (S)
– If you wish to add magnesium, use glyceride, which contains 27% magnesium nutrient (MgO) and 22% sulfur (S).
– If you wish to add calcium, use gypsum, which contains 22-3-% calcium (CaO) and 13-16% sulfur (S).
Micronutrient elements – Plants usually do not require too much micronutrient elements and, with proper planting management, they usually do not show any symptoms. The solutions to lacking micronutrient elements are usually chelation spray through the leaves and soil applications such as adding organic substances using manure and compost.
Toxicity of Plant Nutrients
Plant nutrients may cause toxicity because of the following three factors:
Symptoms Indicating Toxicity of Plant Nutrient Elements
Normally, too much macronutrients would not result in any visible symptoms as they are much needed by the plants, except in cases where such elements are excessively added.
– With too much nitrogen (N), the leaves of the plants become dark green. Branches get too big and fracture and break easily. Flowering and fruiting are decreased, and the plants become vulnerable to diseases and insects.
– With too much phosphorus (P). the veins on young leaves become yellow. The edges are dried up and the plants show signs of stress and become vulnerable to diseases.
– With too much potassium (K), the plants will show signs of a lack of other nutrients, such as calcium and magnesium.
For secondary macronutrient elements such as calcium (Ca), magnesium (Mg) and sulfur (S): when plants receive too much calcium, they show signs of a lack of potassium and magnesium. On the other hand, if they get too much magnesium, they show signs of a lack of potassium and calcium. Meanwhile, the lack of sulfur will result in a stall in photosynthesis and chlorophyll deformation.
Seven micronutrient elements, namely iron (Fe), manganese (Mn), boron (B), molybdenum (Mo), copper (Cu), zinc (Zn) and chlorine (Cl), we usually come across toxicity more easily than primary and secondary macronutrient elements, because plants require less of those. If the soil has an abnormal pH or receive too much nutrients, toxicity will result in symptoms in the following table:
| Micronutrient Elements | Factors Causing Toxicity | Visible Symptoms |
| Boron (B) | Excessive addition | Pale and dried up leaves from the end or edges |
| Copper (Cu) | Contaminated in the soil or excessive addition | Pale and dying old leaves, stalled root growth |
| Chlorine (Cl) | Seaside soil with limited drainage and salty soil areas | Burnt and dried up leaves and stalled growth |
| Iron (Fe) | Water-logged lowlands | Rice leaves turn yellow and red, or purple in other types of plants |
| Manganese (Mn) | Water-logged lowlands | Brown spots on the veins, dried up veins from the ends and edges, curled up, wavy leaves |
| Molybdenum (Mo) | Too much marl or dolomite to increase the pH, causing molybdenum to become highly dissolvent | Golden yellow to orange yellow leaves, purple in some places, short stalks |
| Zinc (Zn) | Greenhouse or attic plants | Less visible symptoms, similar to lacking iron and manganese |
From table 3-21, page 122: Soil Fertility Kit
References:
ยงยุทธ โอสถสภา, อรรถศิษฐ์ วงศ์มณีโรจน์, ชวลิต ฮงประยูร ปุ๋ยเพื่อการเกษตรยั่งยืน สำนักพิมพ์มหาวิทยาลัยเกษตรศาสตร์, 2551 กรุงเทพฯ (519 หน้า)
ยงยุทธ โอสถสภา, ธาตุอาหารพืช, สำนักพิมพ์มหาวิทยาลัยเกษตรศาสตร์, 2543 กรุงเทพฯ (424 หน้า)
Thomas Dierolf, Thomas Fairhurst and Ernst Mutert Soil Fertilizer Kit Printed by Oxford Graphic Printers, 2001 (149 pp)